Energy Bands in Solids
In the case of insulators, there is no effective force electron. All bands are completely full in the
valence bands, and the conduction band is completely empty and there is large
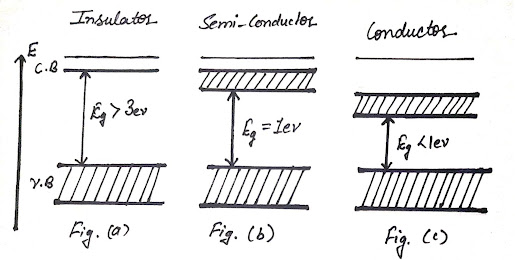
forbidden energy gap![]() between these two bands as shown in Fig. (a) and it is impossible
to excite an electron across this region. All the bands are either completely
filled or empty at any temperature. So the external electric field can not
produce any current. So the conductivity of such materials under ordinary
conditions is zero and are called insulators. A representative example of an insulator is a diamond where two bands separated by an energy gap of
between these two bands as shown in Fig. (a) and it is impossible
to excite an electron across this region. All the bands are either completely
filled or empty at any temperature. So the external electric field can not
produce any current. So the conductivity of such materials under ordinary
conditions is zero and are called insulators. A representative example of an insulator is a diamond where two bands separated by an energy gap of![]() .
.
When the forbidden gap
is small, the order of 1eV
The energy band
structure in solids have two possibilities
1. A solid is a
conductor if either its conduction band is not completely filled and the
valence band may be completely filled and there is an extremely small energy gap
between them as shown in Fig. (c) For example Li, Na, K, etc.
2. The valence band is
completely filled and the empty conduction band overlap with the valence band (For
example Ba, Cd, Zn, etc.) So the energy gap is zero. The electron in the valence
band is free to move inside the crystal lattice. The electrons under the
influence of a small applied field acquire additional energy and move to a higher energy
state. These mobile electrons constitute a current.
For Kronig-Penney Model - Click Here
For Direct and Indirect bandgap in semiconductor - Click Here






2 Comments
You have provided valuable data for us. It is great and informative for everyone. Keep posting always. I am very thankful to you. Energy consumption in Australia
ReplyDeleteHi there to everyone, the contents present at this web page are actually amazing for people knowledge, well, you can also visit Semiconductor Linear Motor. Keep up the good work.
ReplyDeleteIf you have any doubt, please let me know