Sommerfield‘s Quantum Theory
In 1928, Sommerfield suggested that electron gas should be
treated quantum mechanically in the light of Pauli's exclusion principle which
states that no two particles can have all the quantum numbers identical. He
used Fermi-Dirac statistics rather than Maxwell-Boltzmann statistics and
succeeded in explaining many experimental facts. We first find the possible
energy states in one-dimensional and then extend the idea to three-dimensional
case.
Free Electron Gas in one-dimensional Box
Consider an electron
having mass m confined to move in a one-dimensional rectangular box of length L
i.e., The electron moves along a straight line say along the x-axis, let the wall of
the box be rigid and non-penetrable. Let the electron can travel along the x-axis
between x=0 and x= L.
The potential V is
defined as
Or
Where
 ...(2)
...(2)
The general solution of
Eq. (1) may be written as
Where A and B are the constants to be determined by the boundary conditions.
Boundary condition
The wave function Ψ=0 at x=0 and x=L
1. At x=0, Ψ=0, so eq. (3)
becomes
0= 0+B
B=0
So eq. (3) becomes
Ψ= A Sin Kx
2. At x=L, Ψ=0, so eq. (4)
becomes
For nth state eq. (4) becomes
Using eq. (2), eq. (5) can be written as
Or
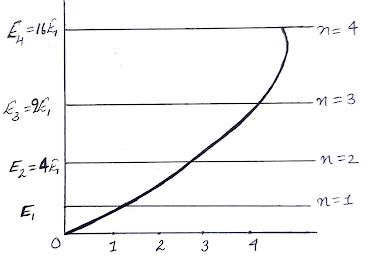
Since E depends on ‘n’,
so let us denote the energy of the electron by En ...(7)
...(7)
The number n is called
the quantum number. We find the lowest energy is obtained when n=1
From eq. (7), we have
i.e.,
Now, the spacing between the energy levels can be found by
That’s mean energy
level separation increases with n.
Fermi Energy
At T=0K, there will be
no thermal excitation i.e., all the electrons remain at ground level. For a large
value of n, there will be the uppermost filled level at T=0K called the Fermi level.
This is the last filled level. The energy of the Fermi level is called Fermi Energy.
In a linear solid,
there are two quantum numbers of a conduction electron orbital i.e., n
(=1,2,3…) and spin s (1/2)
Let nF
denotes the uppermost filled energy level, and if there are N electrons we can
write
Hence eq. (9) give
This is the Fermi
energy in 1-Dim.
Total Energy or Ground
state Energy –
At T=0K, all the
electrons are in ground state i.e., there is no thermal excitation. Sum of all
the energy states will be total ground state energy.
Above eq. can be
written as
As we know

Thus, we find that for one-dimension, the average energy in the ground state is one-third of that of the Fermi energy.
Some Important formulae
for Numerical problem-
From eq. (5), eq.
(10) can be written as
If the electron is free
then its whole energy will be Kinetic energy
i.e.,














































1 Comments
Thanku
ReplyDeleteIf you have any doubt, please let me know